Shop Products
Altogen Laboratory Research Services, Biology CRO Services
Generation of Stably Expressing Cell Lines in 28 Days
We offer generation of stable cell lines by transforming your cell line (or one of our 120 in-house cell lines and primary cells) to stably express vector or gene of interest. Plasmid DNA construct should encode at least one antibiotic resistance gene to enable drug selection. Transfected genetic material can be expressed in the target cells either transiently or permanently depending on the methods utilized and the experimental questions being investigated.
RNAi Services
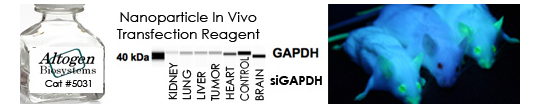
We provide all types of RNAi services from siRNA design to biodistribution and imaging studies in vivo. Our services include siRNA liposome encapsulation, quantitative measurement of RNAi effect on mRNA and protein expression levels, in vivo validation, tissue targeting, etc. RNA interference (RNAi) is a phenomenon by which the expression of double stranded RNA (dsRNA) specifically stimulates a cellular process that reduces gene expression in a sequence specific manner. Small synthetic RNA, termed small interfering RNAs (siRNA), which are typically 21-28 nucleotides in length, can induce RNAi and knockdown gene expression in mammalian cells without inducing an antiviral response.
MTS/PMS In Vitro Cytotoxicity Assays and IC50 for Tumor Cell Lines
We offer a number of cell proliferation / cell cytotoxicity assays, IC-50 assay in over 120 cell lines and primary cell types, flow cytometry based assays (cell cycle, cell viability, membrane protein expression, TUNEL apoptosis, etc) and many other cell based assays. Standard cytotoxicity assays are based on the cleavage of the yellow terazolium salt (XTT) by metabolically active human cells (A549, HepG2, or HeLa) to form an orange formazan dye. This conversion only occurs in viable cells, so cytotoxicity leads to a loss of activity. The formazan dye formed is soluble in aqueous solutions and is directly quantified using a scanning multi-well spectrophotometer. Using multiple experimental sample replicates (experiment conducted in 96 well plates) this method ensures a high degree of accuracy with statistically significant results demonstrating biological activity of test compound.
Phosphodiesterase, Phosphatase, Kinase, and ELISA Assay Development
We have over 15 years expertise in ELISA assay development and validation. This service is offered as both non-GLP (research purposes) and GLP-compliant service for diagnostics. The kinase, phosphatase, and phosphodiesterase assay development services are usually utilized for screening and validation studies. For each project, an individual pathway is defined that matches the biology and chemistry requirements of the target to the most appropriate assay technology platform available. Our company scientists have experience developing assays with all major fluorometric readouts and biologically relevant formats.
Immunohistochemical Staining (IHC Assay Development Service)
We provide both automated Western Blot protein expression services and immunohistochemical staining services that are available for cell culture projects, as well as for frozen tissue samples, formalin fixed samples, paraffin-embedded tissue blocks, or unstained slides. Our company scientists can process the specimens, perform antibody staining test or develop IHC staining protocol. Total RNA, DNA, and protein isolation services are also available.
Custom Preclinical Research Services
Other laboratory research services include:
- Over 50 validated xenograft models – link
- Assay development (ELISA, RT-PCR, Western Blot) and library screening – link
- Pharm/tox and safety IND tests – link
- Generation of stable cell line in 28 days – link
- RNAi experiments (siRNA design, synthesis, validation) – link
- Liposome encapsulation service – link
Nonclinical studies typically involve the following steps:
- In vitro studies: In vitro studies are conducted in laboratory settings using cells or tissues to test the safety and efficacy of the treatment.
- Pharmacokinetics and toxicology: Researchers conduct pharmacokinetics and toxicology studies to evaluate how the treatment is absorbed, distributed, metabolized, and eliminated by the body, as well as to assess any potential toxic effects.
- Animal studies: Animal studies are conducted to evaluate the safety and efficacy of the treatment in vivo, typically using several animal species to provide a comprehensive assessment of the potential risks and benefits.
- Formulation and manufacturing: Researchers also develop and optimize the formulation and manufacturing processes for the treatment to ensure consistency and quality.
The results of nonclinical studies are then used to support the design of human clinical trials, which are conducted in several phases to further evaluate the safety and efficacy of the treatment in humans. If the clinical trials are successful, the treatment may then be approved for use by regulatory agencies and marketed to the public.
Nonclinical research plays a critical role in ensuring the safety and efficacy of new treatments and devices, and in protecting the health and well-being of patients. The nonclinical data generated from these studies is used to make informed decisions about whether a new treatment is safe and effective enough to proceed to human clinical trials.
Featured transfection products from Altogen Biosystems:
HepG2 Cell Transfection Reagent || HUVEC Transfection Reagent || In Vivo Lipid Transfection Kit
Transfection Resource:
Visit Altogen’s Transfection Resource: RNAi applications, stable and transient transfection, delivery methods, electroporation, nanoparticle, microinjection, dendrimer-based, non-viral and virus-mediated gene delivery, liposome and non-liposomal reagents, In Vivo Transfection Reagents from Altogen Biosystems.