Shop Products
Transfection Methods and Techniques
Transfection Methods and Techniques
Transfection is a common laboratory cell culture technique used in many research areas, drug discovery, and development. In vitro transfection refers to the delivery of cargo molecules (such as nucleic acids – DNA or RNA) into cultured cells (usually cancer cell lines). In vivo transfection refers to delivery of cargo molecules (such as therapeutic siRNA, plasmid DNA, small protein, etc) to the target tissue. There are commercially available transfection products optimized for in vitro and in vivo transfection (see transfection kits), as well as transfection services provided by biology CROs (see list of biology CRO companies) that are GLP certified to perform these preclinical experiments per FDA requirements.
Transfection methods include various approaches (physical and chemical methods) that are carried out by non-viral techniques – electroporation, calcium phosphate exposure, liposome-based transfection that allow to deliver cargo molecules through cellular membrane without any permanent damage to the cell. Several chemical transfection methods are described below.
Chemical transfection methods
Chemical transfection is a popular technique due to the ease, cost, and wide variety of transfection reagents available for purchase. Transient transfection is commonly used for short term expression of a desired gene that lasts a few days. Research applications include research of gene expression, gene silencing studies and analysis of recombinant proteins.
The workflow of a chemical transfection experiment is common across cell types. This includes plating cells at subconfluency, preparing the transfection reagent / DNA complex immediately before transfection, and then assessing construct expression. Cell culture medium is refreshed a day after plating cells and then a few hours after transfection. Optimization of the transfection protocol must be undertaken to ensure high transfection efficiency and that the method is not toxic to the cells being transfected.
Lipofection is the transfection using liposomes, small molecules which can fuse with cell membranes and are able to release their contents. Liposomes can be lipid-based (more common), or non-lipid based. Cationic polymers, carrying a positive charge, bind well to negatively charged nucleic acids, and are another method of transfection. There are several common applications of liposome transfection methods used in new medicines discovery and development, especially in oncology where applications include targeting specific genes (e.g. oncogenes) via small molecule-based drugs or gene silencing using RNAi technology.
Physical transfection methods
Electroporation and cell injection (e.g. gene guns) are two classical examples of physical transfection methods that are able to accomplish delivery of nucleic acids, but are harsh on cells due to disruption of the cellular membrane and often results in cell death. It is an appropriate option for cells that have been traditionally difficult to transfect. Physical transfection involves electroporation, microinjection and biolistic particle delivery.
Electroporation is dependent on DNA concentration and the type of cells used. Traditional electroporation requires the use of an electroporation cuvette. Many companies sell electroporation systems which are compatible with cell types ranging from primary and stem cells to prokaryotic and mammalian cells.
Microinjection is commonly used to introduce DNA and RNA into single cells such as embryonic stem cells. With the aid a micromanipulator and microscope, the DNA or RNA is directly inserted into the cytoplasm or nucleus. This is typically time consuming, but results in very high transfection efficiency.
Biolistic particle delivery relies on microparticles carrying nucleic acid to introduce DNA or RNA into cells. A fast method, these particles are shot into cells, but the downside is that there is a high cell mortality rate.
Transfection Protocols and Experimental Details
Although transfection is an incredibly broad and useful technique, it can be highly specific to the cell-type being transfected. Some cells are more amenable to transfection while others are not, and as a result some cells are more responsive to certain transfection reagents. The extensive variety of cell lines and the specific nuances associated with each one would require a textbook to sufficiently describe, but thankfully transfection reagents are out there that can help an experiment function without the need for extensive background research. Many companies develop transfection reagents specifically optimized for given cell types, thus ensuring a maximal efficiency in gene-editing experiments.
When designing a transfection experiment, it is particularly important to understand the final goal of the transfection; stable expression requires a different setup than does transient transfection, and as such, experimental protocols should be adjusted. If time is pressing, then transfection experiments can be outsourced to reliable companies that can guarantee the development of stable or transient cell lines for research applications.
In Vivo Transfection Reagents from Altogen Biosystems
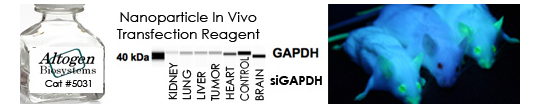
RNAi has been used for in vivo target validation studies using animal models. The major challenge in performing RNAi studies in vivo is the effective, directed delivery of functional small RNA molecules into specific tissues. Altogen® In Vivo Transfection Reagents could be conjugated with siRNA (or microRNA) and administered intratumorally (i.t) or systemically via intravenous (i.v) tail vein injection in order to provide directed gene silencing in specific tissues, including liver, pancreas, kidney, and tumors. Selective knockdown could be seen as early as 24 hours after injection.
Featured in vivo transfection products from Altogen Biosystems:
In Vivo Nanoparticle Transfection Kit || In Vivo Lipid Transfection Kit || In Vivo Pancreas-targeted Transfection Kit
Altogen Custom Services provide specialized biotechnology and pharmaceutical services, including over 60 validated xenograft models, development of stable cell lines, RNA Interference (RNAi) services, assay development, ELISA and Western Blot services, siRNA library screening and transfection services. Generation of stably-expressing cell lines can be very expensive and time-consuming. Altogen Labs offers generation of stable cell line service completed in just 28 days (see service details).
Transfection Methods and Cell Transfection Techniques
Cell transfection is a laboratory technique used to deliver nucleic acids, such as DNA, RNA, or siRNA, into cells to modify their gene expression or to study gene function. The technique involves introducing the nucleic acids into cells using a transfection reagent, which facilitates their entry into the cells.
Cell transfection typically involves the following steps:
- Selection of transfection reagent: Researchers select a transfection reagent that is compatible with the type of cells being transfected and the type of nucleic acid being delivered.
- Preparation of nucleic acids: The nucleic acids are prepared, either by isolating them from natural sources or synthesizing them in the laboratory.
- Formation of transfection complex: The nucleic acids are mixed with the transfection reagent to form a complex that can enter cells.
- Transfection of cells: The transfection complex is added to the cells, and the cells are incubated to allow the nucleic acids to enter the cells.
- Analysis of transfection efficiency: The efficiency of transfection is evaluated by measuring the level of gene expression of the delivered nucleic acid using techniques such as PCR, western blotting, or fluorescence microscopy.
Cell transfection is a valuable tool for studying gene function and for developing new therapies for genetic diseases or cancers. However, the efficiency and specificity of transfection can be affected by various factors, including the choice of transfection reagent, the type of cells being transfected, and the experimental conditions. Therefore, careful optimization of transfection conditions and appropriate controls are important to ensure the reliability and reproducibility of transfection experiments.